News Center
mTOR Inhibitor Developed by Suzhou Kintor Obtained Approval for Clinical Trials in China
mTOR Inhibitor Developed by Suzhou Kintor Obtained Approval for Clinical Trials in China
(Summary description)[Suzhou, August 20, 2019] National Medical Products Administration officially granted a clinical trial approval to Suzhou Kintor Pharmaceuticals, Inc. ("Suzhou Kintor") on commencement of clinical trials of the drug (GT0486) as an mTOR inhibitor. The drug (GT0486) is a novel mTORC1 / mTORC2 inhibitor (the second generation of mTOR inhibitor) which is indicated for advanced solid tumors. Currently, there is no second generation of mTOR inhibitors on the market in China, and there are huge unmet clinical needs in the research area.
- Categories:Company news
- Author:
- Origin:
- Time of issue:2019-08-20 14:30
- Views:
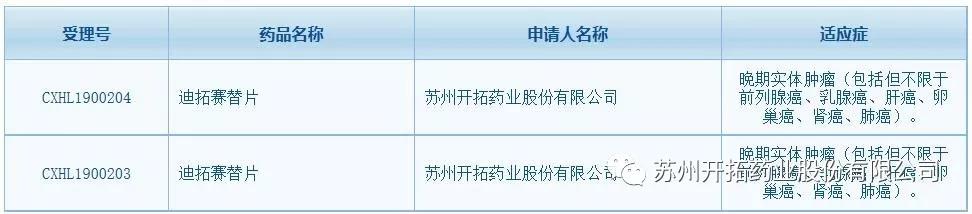
The mTOR inhibitors developed by Suzhou Kintor has been approved for clinical trials, which signifies that drug (GT0486), as the second generation of selective small molecule inhibitor of mTOR with a global patent independently developed by the Group, has officially proceeded into the clinical development from pre-clinical development, and the continuous expansion of the clinical innovative pipeline drugs from the Group.
About Suzhou Kintor
Founded in March 2009, Suzhou Kintor is an innovative enterprise and industrialized platform committed to the development of “best-in-class” and “first-in-class” drugs. Suzhou Kintor's products are Class 1 novel drugs covering the prostate cancer, breast cancer, liver cancer and hair loss, and are granted more than 50 patents worldwide. Many projects have been listed as the National Special Project for "Significant Novel Drugs Development" during the 12th and 13th Five-Year Plan period. Its major product, Proxalutamide, is undergoing clinical trials in China and the United States concurrently. Clinical trials of this product are conducted as a second-line therapy and a first-line therapy for mCRPC in the phase III in China and in the phase II in the United States. Phase I/Ib clinical trials have been completed in China for metastatic breast cancer, and Phase Ic clinical trials for combined drugs are ongoing for HR+/AR+/HER2- breast cancer; Phase I clinical trials of KX-826 for androgenetic alopecia have been completed in China and the United States, and phase II clinical trials are ready to be carried out in China; ALK-1 monoclonal antibody is the first project to be granted global development rights for novel drugs against tumor by Pfizer to Chinese companies. Two phase I clinical trials in more than 100 subjects have been completed in the U.S. and other regions. A phase II clinical trials is currently being conducted in Taiwan for ALK-1 monoclonal antibody combined with PD-1 (Nivolumab) for the treatment of liver cancer. The R&D pipeline also includes Hedgehog / SMO inhibitors, cMyc inhibitors and AR-Degrader. Suzhou Kintor was selected as the "Unicorn Cultivation Enterprise" in Suzhou City and Suzhou Industrial Park in 2018, respectively.
Business
News Center
Contact Us
Find Us


Copyright: Kintor Pharmaceutical Limited retains all copyrights. 苏ICP备19028699号-2 Powered by 300.cn
Complaints and Suggestions Tel: +86 512 62639935 Email: public@kintor.com.cn




