Recently, a group of data indicating more than 250 million people with hair loss in China has circulated online and caused heated discussion. In fact, hair loss is a common disease worldwide. Under normal circumstances, a healthy person will have nearly a hundred hairs shed naturally every day, and an equal amount of hair will be reborn from the scalp hair follicles. If hair loses faster than growth of newborn hair, there will be hair loss problem, which will affect the aesthetics and mental health.
There are many reasons for hair loss. Hair loss caused by external factors such as trauma, infection, and medication (chemochemic hair loss) accounts for only a small part, and more than 90% is a result of androgenic alopecia. This is because human hair follicles are very sensitive to dihydrotestosterone(DTH), a natural metabolite of androgens in the body. DHT can promote the development of male sexual characteristics (such as facial hair) during adolescence, but after adulthood, excessive DHT in the body can produce negative effects, such as causing whelk, acne, enlarged prostate and androgenic alopecia. Adult men and women may lose hair due to changes in androgen metabolism in the body, especially men.
There are very limited drug treatment means for hair loss. To date, the FDA has only approved 2 drugs for androgenic alopecia, namely minoxidil and finasteride. The most recent finasteride was even approved for marketing in 1992. GlaxoSmithKline's dutasteride was approved for treatment of androgenic alopecia only in Japan on September 28, 2015.
Both finasteride and dutasteride on the market are 5α reductase inhibitors, which inhibit the activity of 5α-reductase to reduce DHT production, thereby treating androgen-dependent diseases, including androgenic alopecia, acne, etc. According to the NextPharma data of Pharmcube, other androgenic alopecia drugs under development are mostly in the early and middle stages before phase II, and there are diverse mechanisms of action, including WNT signaling pathway regulator SM04554, JAK inhibitor ATI-50002, androgen receptor antagonist clascoterone, CRTH2 antagonist setipiprant, thyroid hormone receptor agonist PF-00277343 and so on.
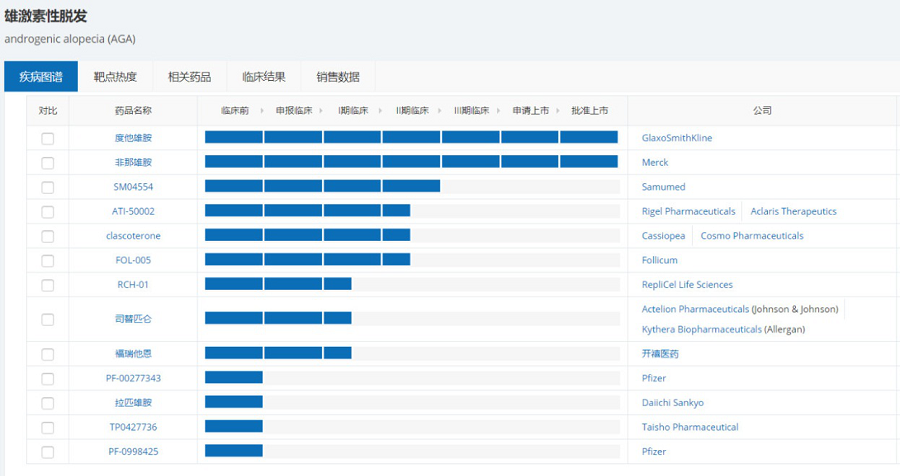
Source:NextPharma of Pharmcube
Where,the most noteworthy one is the androgen receptor antagonist clascoterone developed by an Italian company,Cassiopea,which is currently in phase III and phase II development for indications of acne and androgenic alopecia,respectively. Clascoterone has clear mechanism of action,whose innovation is that it is a pioneering androgen receptor inhibitor that acts on local skin. It can penetrate the skin to reach the androgen receptor in the sebaceous glands and hair follicles,thereby inhibiting DHT effect of local skin. Compared with oral finasteride for systemic absorption,on the one hand,it can work locally and accurately to avoid the growth of hair (such as eyebrows and beards) outside the hair loss area,which is very important for men and women with hair loss. On the other hand,it reduces systemic side effects,thus safer and more effective.
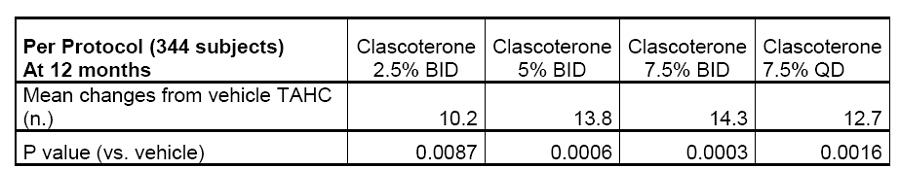
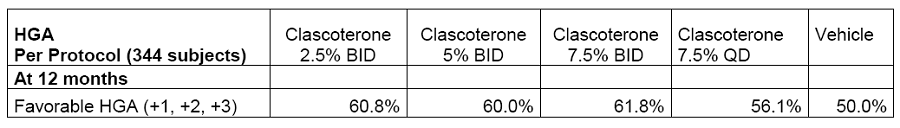
On July 10,2018,Cassiopea announced positive results achieved in the two key phase III studies (Study 25 and 26) of clascoterone 1% cream for acne treatment. The two studies enrolled 1,440 acne patients over 9 years of age (IGA scored 3 or 4),who were randomly grouped and given clascoterone 1% cream or placebo twice daily for 12 weeks. After completion of the study,604 patients entered a long-term open-label study,who were continually administered for 12 months to evaluate the safety of clascoterone. The results showed that the proportion of patients with IGA scores reduced by more than 2 points in the intent-to-treat population significantly increased in both studies (16.1% vs 7.0%,16.7% vs 6.5%),reaching the primary endpoint. Based on the results of these two studies,Cassiopea just submitted a marketing application for clascoterone 1% cream in acne treatment to the FDA on August 22. On April 16,2019,Cassiopea announced the 12-month results of the Phase II study on clascoterone in androgenic alopecia treatment. Among 400 male androgenic alopecia patients aged 18 to 55 who were enrolled in Germany,after 12 months’ treatment with Clascoterone cream of different concentrations,target area hair count (TAHC) and hair growth score (HGA) were significantly improved compared with the control group.
The drug under development by domestic enterprise with similar mechanism of action and usage as clascoterone is KX-826 of Suzhou Kintor ,which is a tincture locally administered as qd or bid in clinical trials. KX-826 is actually the second fastest developing androgenic alopecia treatment drug after clascoterone for local administration in the world,which ranks first in development speed domestically. According to the public information,KX-826 gained clinical approvals in China and the United States in 2018. So far,Phase I and Ib clinical trials have been completed in China,and phase Ib clinical trials are going on in the United States. 102 subjects participated in the clinical trials. The corresponding PK and safety data have been obtained. On July 26,Suzhou Kintor held a phase II clinical research protocol discussion meeting in Beijing on KX-826 for the treatment of androgenic alopecia (AGA) in Chinese male adults. Considering that clascoterone has submitted a marketing application to the FDA and the market value of Cassiopea has been steadily rising,KX-826 development progress of Suzhou Kintor is also worthy of attention.
In short,the domestic hair loss population is huge,and the industries such as hair transplantation and wigs are enjoying booming development. Various shampoos for hair loss treatment are popular,but the truly safe and effective drug treatment means are far from meeting the needs. The global sales peak of MSD’s finasteride is only USD447 million,which is mainly related to the limitations of the product itself. So far,the ongoing projects for hair loss have big differences in the mechanism of action. Hair loss drugs are in the blue ocean market with good competition pattern. We will subsequently pay close attention to the progress of global novel drugs development in this field.









